티스토리 뷰
[Potent Irreversible GST Inhibitor] CNBSF [FDV-0031][CAS No. 3829-23-0]_Funakoshi - 코아사이언스
코피디 2024. 3. 18. 16:19
CNBSF
Potent Irreversible GST Inhibitor
Cat.# FDV-0031 / Size: 10 mg
CNBSF (2-Chloro-5-Nitrobenzen Sulfonyl Fluoride) is an irreversible inhibitor for glutathione S-transferases (GSTs). CNBSF directly binds to GSTs and inactivates their enzymatic activity. Compared with conventional reversible inhibitors, CNBSF could inhibit GST activity for long term.
After treatment of CNBSF to CHO cells to inhibit intracellular GST activities, remaining GST activities in the cells were measured by ☞ CellFluor™ GST, a fluorescence probe for GST in cells. CNBSF dramatically suppressed the fluorescent signal from cells.
GST and its Inhibitors
Function of GSTs
Glutathione S-Transferase (GSTs) are widely conserved in nature from bacteria to plants and animals. In human, over 20 members are identified and classified into three categories: cytosolic, mitochondrial, and membranebound microsomal members. GSTs are phase-II detoxification enzymes and commonly play an important role in detoxification of hydrophobic and electrophilic compounds including endogenous toxic metabolites or xenobiotics by conjugating with glutathione (GSH) to produce glutathione-conjugate (GS-conjugates). Generally, GSTs have two types of substrate-binding site, called G-site and H-site, for GSH and hydrophobic substrate (xenobiotics), respectively. When GSTs bind to GSH as the first substrate, GSTs catalyze and stabilize thiol group of GSH as thiolate anion. Once hydrophobic and electrophilic xenobiotics bind to GSTs as the second substrate, GSTs transfer them to GSH to form GS-conjugates. GS-conjugates are released from GSTs and quickly exported to extracellular space by multidrug resistance-associated protein (MRP) transporters. Through the above processes, GSTs detoxify toxic compounds.
As many studies suggested expression level of GSTs are significantly increased in cancer cells, GSTs are considered as anticancer drug-resistant enzymes in malignant cancer cells through the neutralization of drugs. Inhibition of GST activities is one of the promising strategies to improve drug efficiency in cancer cells. Some GST inhibitors have been developed so far, however, conventional inhibitors including ethacrynic acid (EA), a representative GST inhibitor, were based on the competition with GSH. In general, competitive inhibitors are usually reversible and not sufficient in cells because of high concentration around mM order of GSH in cells. Although several irreversible inhibitors were also discovered and show potent inhibition in vitro, these compounds have low membrane-permeability and are not good at live cell experiments. To overcome conventional problems, highly membrane-permeable and irreversible inhibitors are desired.
Novel Strategy for Irreversible GST Inhibition
2-chloro-5-nitrobenzensulfonyl fluoride (CNBSF) is a novel type of irreversible GST inhibitor reported in 2019 1. CNBSF is membrane-permeability and capable of entering into cytosol. Once CNBSF incorporated into cell, GSTs catch CNBSF in H-site as a hydrophobic xenobiotics. Subsequently GSTs convert CNBSF to GS-conjugated CNBSF, called GS-5NBSF. In the case of human GSTP1, a member of π-type GST, Tyr108 residue in human GSTP1 quickly reacts with sulfonyl fluoride group of GS-5NBSF, fluoride anion was leaved, and form covalent bound between GST and substrate. In the result of above scheme, GSTs covalently bound inhibitor-complex are inactivated.
Principle
Proposed Reaction Mechanism of CNBSF to GSTs
Ref.1 proposed the reaction mechanism of CNBSF in the case of human GSTP1.
- GSH and CNBSF are bound to G-site and H-site in GST, respectively. Tyr 7 of GSTP1 stabilizes thiolate anion of GSH and the thiolate anion of GSH attacks to CNBSF.
- Chloride anion leaves from CNBSF and GS-5NBSF is formed.
- Subsequently, alkoxide anion of Tyr 108 attacks to sulfonyl fluoride group.
- Ternary complex is formed.
Features
- CNBSF is a membrane-permeable compound and can be used in live cell experiments. For in vitro assay, addition of GSH is essentially required.
- CNBSF directly binds to the catalytic site of GSTs and inactivates GST function for long time. Compatible with both in vitro and in cellulo assay.
- Cytosolic GSTs including GSTα, GSTμ, GSTπ, GSTω, GSTθ and GSTζ were experimentally validated targets of CNBSF *.
- *Note : Membran-bound GSTs are not validated at the moment.
- Conversion of CNBSF to GS-5NBSF in cells is experimentally validated by mass spectrometry 1.
- In combination with ☞ CellFluor™ GST, a cell-based GST activity assay reagent, GST inhibitory activity in living cells can be quantitatively evaluated.
- CNBSF is a pro-drug of GST inhibitor. By being added to GSH by GST in the cell, it functions as a GST inhibitor (GS-5NBSF).
Note for Experimental Setting
CNBSF can be stored in DMSO stock solution for long-term in less than -20℃. However, CNBSF shows highly chemical reactivity in aqueous solutions. Especially in culture media such as DMEM, CNBSF quickly reacts with various components of DMEM, resulting in its decomposition. For example, a stability of CNBSF in serum-free DMEM at 37℃ is experimentally estimated, 60% degradation in 10 min and ~95% degradation in 30 min. Therefore, the use of inorganic salt buffers such as HEPES-buffered saline is recommended for live cell experiments. Hydrolysis of sulfonyl fluoride group of CNBSF is still observed gradually even in HBS (~50% hydrolysis in 30 min, ~70% hydrolysis in 60 min at 37℃ in HBS). So, working solution in aqueous solution such as HBS should be prepared just before use to avoid hydrolysis of CNBSF. Based on degradation or hydrolysis of CNBSF in buffer or medium, long-term incubation (>1 hour) of CNBSF for cells may not effectively. If the use of medium is required for your experiments, please optimize CNBSF concentration such as 1 mM.
Application Data
- ☞ Broad Inhibition Activity for Cytosolic GST Members
- ☞ Irreversible Inhibition activity in vitro
- ☞ Irreversible Inhibition activity in cellulo
- ☞ Quantification of Inhibitory Activity of CNBSF in cellulo
- ☞ Comparison of Intracellular GST Inhibition Between CNBSF and Rthacrynic acid (EA) in cellulo
- ☞ Estimation of Recovery Time of GST Expression
- ☞ CNBSF Promoted Production of Lipid-peroxidation (LPO) Downstream Metabolites
Broad Inhibition Activity for Cytosolic GST Members
Recombinant human or mouse cytosolic GST enzymes and reduced GSH (10 μM) were added into assay buffer (50 mM sodium phosphate (pH 7.4), 150 mM NaCl). DMSO for the control experiment or CNBSF (100 μM) was further added to them and incubated for 30 min. After then, a fluorescent GST activity assay reagent ☞ CellFluor™ GST (final 5 μM) was added and incubated for 30 min. Fluorescent intensity of each sample was measured by fluorescent plate leader (Ex 475±5 nm/Em 525±10 nm).
Irreversible Inhibition activity in vitro
Recombinant human GSTM1 or GSTA1, GSH (final 100 μM) and CNBSF (final 100 μM) were added to PBS and incubated for 30 min at 37℃. Each sample was split into two vials and one vial was applied into micro-dialysis kit (1 kDa-cut off) for 30 min in PBS. After dialysis, reduced GSH (additionally 100 μM) and ☞ CellFluor™ GST (1 μM) were further added to each sample and incubated for 30 min.
Neither GSTM1 nor GSTA1 activity was restored by dialysis after addition of CNBSF.
Irreversible Inhibition activity in cellulo
CHO cells were seeded in 96 well plate at 1×104 cells/well and cultured in DMEM containing 10% FBS for 20 hours. Then culture media were replaced with HBS, treated with 100 μM CNBSF and ☞ CellFluor™ GST in 3 different protocols shown in the right scheme. The fluorescent intensities were measured with a fluorescent plate reader (Ex 475±5 nm/Em 525±10 nm).
In all protocols, GST activities were highly suppressed, indicating that CNBSF irreversibly inhibits intracellular GSTs.
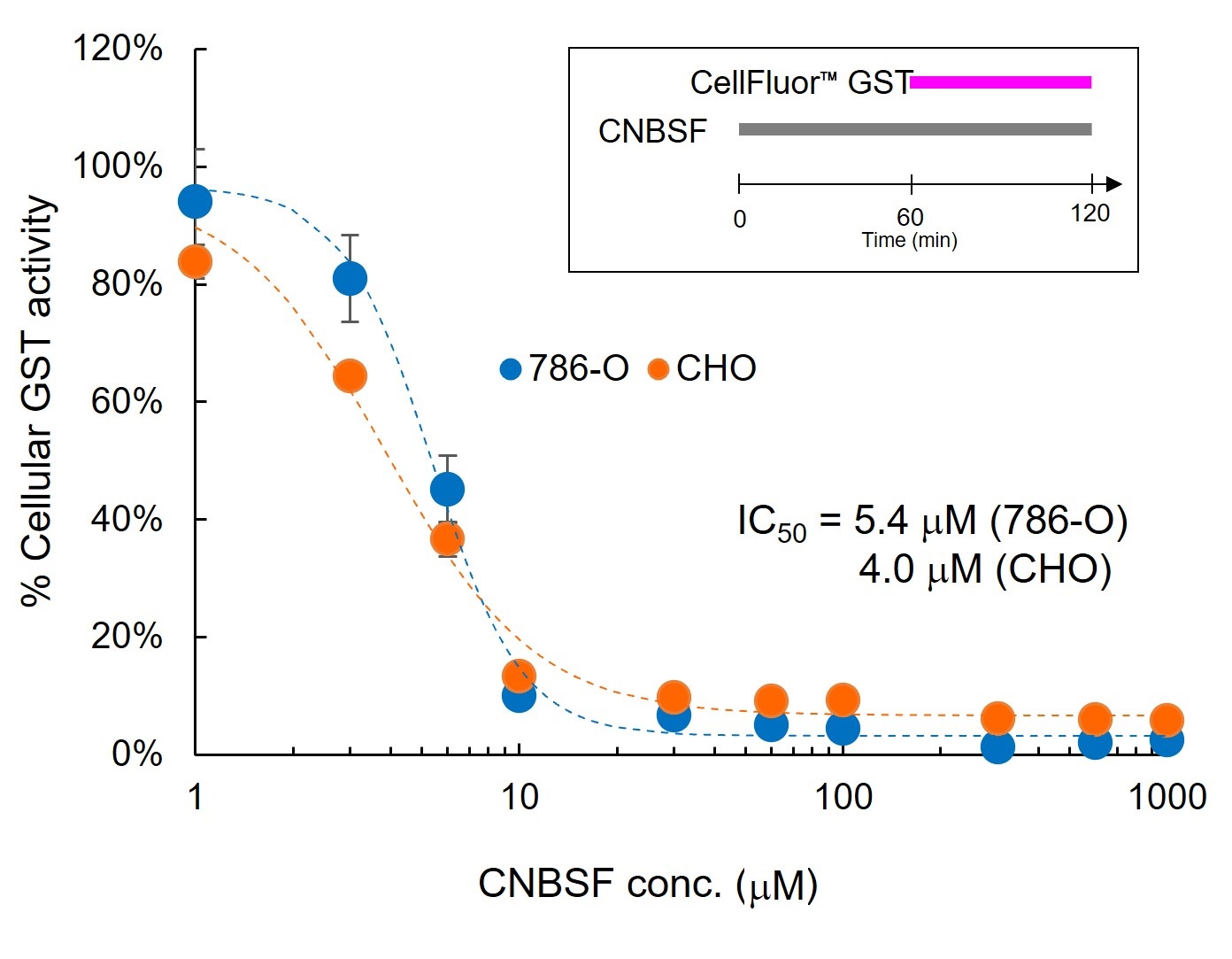
Quantification of Inhibitory Activity of CNBSF in cellulo
786-O and CHO cells were seeded in 96 well plate at 1×104 cells/well and cultured in DMEM containing 10% FBS for 20 hours. Then culture media were replaced with HBS, treated with 1-1000 μM CNBSF for 1 hour, and further treated with 2 μM ☞ CellFluor™ GST for 1 hour. The fluorescent intensities were measured with a fluorescence plate reader (Ex 475±5 nm/Em 525±10 nm).
Both 786-O and CHO cell lines showed similar IC50 values around 4-5 μM in cell-based assay.
Comparison of Intracellular GST Inhibition Between CNBSF and Rthacrynic acid (EA) in cellulo
786-O and CHO were seeded in 96 well plate at ×104 cells/well, cultured in DMEM containing 10% FBS for 20 hours. Then culture media were replaced with HBS, cells were incubated in the presence of 20 μM CNBSF or ethacrynic acid (EA) for 1 hour, and further treated with 2 μM ☞ CellFluor™ GST for 1 hour. Fluorescent intensities were measured with a fluorescent plate reader (Ex 475±5 nm/Em 525±10 nm) without medium change.
In both 786-O and CHO cells, CNBSF showed higher GST inhibitory activity than ethacrynic acid at 20 μM concentration.
Estimation of Recovery Time of GST Expression
786-O and Neuro2a were seeded in 96 well plate at 1×104 cells/well, and cultured in DMEM containing 10% FBS for 20 hours. Then the culture media were replaced with serum- and phenol red-free DMEM (DMEM(−)). After incubation in HBS containing 100 μM CNBSF for 20 min, the HBS media were returned to DMEM(−) and cultured for 0, 2, 4, and 6 hours. The cells were then simultaneously treated with DMEM(−) containing ☞ CellFluor™ GST (2 μM) for 1 hour. The fluorescent intensities were measured with a fluorescent plate reader (Ex 475±5 nm/ Em 525±10 nm).
In 786-O cells, the inhibitory effect of GST was observed to continue for at least 6 hours, while a gradual recovery was observed over time for Neuro2a cells, indicating prompt rescue expression of GSTs.
CNBSF Promoted Production of Lipid-peroxidation (LPO) Downstream Metabolites
CHO cells were seeded in 96 well plate at 1×104 cells/well and cultured in DMEM containing 10% FBS for 20 hours. Then culture media were replaced with HBS and pretreated with 100 μM CNBSF for 1 hour. Cells were then washed with PBS, subsequently treated with lipid-peroxidation (LPO) initiators, H2O2 (0.1 mM or 0.5 mM), nitric oxide (NO) donor (SNAP; 0.1 mM), or radical initiator (APS; 1 mM) in HBS for 20 min or Cu2+ (CuSO4; 1 mM) in HBS for 5 min. Cells were washed with HBS and ☞ AcroleinRED (2 μM), a cell-based detection reagent for acrolein, or TAMRA fluorescent dye as a negative control in HBS was treated for 30 min. Then, cells were washed 3 times with HBS to remove unreacted ☞ AcroleinRED or TAMRA, and fluorescent intensities were measured with a fluorescent plate reader (Ex 540±5 nm/ Em 580±10 nm).
Under the control condition, each drug slightly increased acrolein production. Pretreatment of CNBSF dramatically increased acrolein production and CNBSF with each LPO initiators synergistically promotes acrolein production. These results suggest the physiologically generated or drug-induced acrolein was suppressed by the cellular GST activities.
Reference
- Shishido, Y., et al., "A Covalent Inhibitor for Glutathione S-Transferase Pi (GSTP1-1) in Human Cells." ChemBioChem, 20(7)、900~905 (2019). [PMID:30548113]
* Research use only, not for human or animal therapeutic or diagnostic use !
'실험.연구용 추천상품' 카테고리의 다른 글
- Total
- Today
- Yesterday
- 조직절편제작
- 면역화학분석
- Funakoshi
- 파라핀 블록
- 오토클레이브백
- OPV
- Sterlitech
- 건스터바이오텍
- 막필터
- 바이오헤저드백
- Coresciences
- 바이오하자드백
- time release pellets
- 조직절편
- 후나코시
- 형광염색
- 조직염색절편제작
- 콘드로이틴 황산 올리고당
- gradient gel
- matrix-driven delivery pellet
- 파라핀 블럭
- filter
- material science
- 미니멸균백
- OLED
- solar cells
- 연속절편
- 고수용성 콘드로이틴
- allview PAGE buffer
- 코아사이언스
| 일 | 월 | 화 | 수 | 목 | 금 | 토 |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| 27 | 28 | 29 | 30 |