티스토리 뷰
[GST Enzymatic Activity Assay Reagent for Cell-based High Through] CellFluor™ GST [FDV-0030]_Funakoshi - 코아사이언스
코피디 2024. 3. 18. 14:39GST Enzymatic Activity Assay Reagent for Cell-based High Through
CellFluor™ GST
Cat.# FDV-0030 / Size: 0.1 µmol
CellFluor™ GST is an novel fluorescent probe for measuring activity of glutathione S-transferase (GST) that can be applied to living cells. It is rapidly taken up by cells and emits green fluorescence due to intracellular GST activities, making it useful for the evaluation of GST activity on live cells. The protocol does not require any washing before fluorescent measurement, and only the addition of the reagent to the culture medium is required and makes it easy to construct a high-throughput screening system using a fluorescent plate reader. It can also be used for in vitro assays such as cell or tissue lysates, and has the advantage of higher detection sensitivity compared to conventional reagents.
CellFluor™ GST는 살아있는 세포 내에서 Glutathione S-Transferase (GST) 활성을 측정하기 위해 개발된 새로운 형광 프로브입니다.
|
Notice: Product Name Changes
This product has been renamed as follows:
|
CellFluor™ GST is a quenched fluorescent dye containing two GST-responsive protecting groups on Rhodamine 110, and has excellent cell permeability. The removal of the protecting group by various GSTs in the cell exhibits green fluorescence of Rhodamine110. By measuring the green fluorescence intensity with a fluorescence plate reader or fluorescence microscopy, GST activity can be evaluated on live cells.
GST and its Analytical Methods
What is GSTs?
Glutathione S-Transferase (GSTs) are widely conserved in nature from bacteria to plants and animals. In human, over 20 members are identified and classified into three categories: cytosolic, mitochondrial, and membrane-bound microsomal members. Cytosolic GSTs consist of 6 subfamilies including α (GSTA), μ (GSTM), π (GSTP), θ (GSTT), ο (GSTO) and ζ (GSTZ). Mitochondrial member is κ (GSTK) and microsomal members are MGSTs and membrane associated proteins in eicosanoid and glutathione metabolism (MAPEGs). GSTs are phase-II detoxification enzymes and commonly play an important role in detoxification of hydrophobic and electrophilic compounds including endogenous toxic metabolites or xenobiotics by conjugating with glutathione (GSH) to produce glutathione-conjugate (GS-conjugates) (Figure 1). Generally GSTs have two types of substrate-binding site, called G-site and H-site, for GSH and hydrophobic substrate (xenobiotics), respectively. When GSTs bind to GSH as the first substrate, GSTs catalyze and stabilize thiol group of GSH as a thiolate anion. Once hydrophobic and electrophilic xenobiotics bind to GSTs as the second substrate, GSTs transfer them to GSH to form GS-conjugates. GS-conjugates are released from GSTs and quickly exported to extracellular space by multidrug resistance-associated protein (MRP) transporters. Through the above processes, GSTs detoxify toxic compounds.
As many studies suggested expression level of GSTs are significantly increased in cancer cells, GSTs are considered as anticancer drug-resistant enzymes in malignant cancer cells through the neutralization of drugs.
Conventional Problems and CellFluor™ GST Assay
To understand biological functions of GSTs as phase-II detoxification enzymes, research tools for monitoring GST activity are very important. Although several reagents including a classical GST substrate CDNB (1-chrolo-di-nitrobenzen) for this purpose have been developed, the probes which can be applied into measurement of intracellular GST activity are highly limited. To monitor physiological function of GSTs, the tool for live cell-based GST activity assay is desired. CellFluor™ GST, another name DNs-Rh or bis-DNs-Rh (ref.1-4), is a Rhodamine 110 derivative which protected by DNBs (dinitrobenzenesulfonamide). This probe shows very low fluorescence (quantum yield = 0.0007). After deprotected by GSTs via coupling of DNB-glutathione conjugates, Rhodamine 110 was released and emits strong green fluorescence (quantum yield = 0.645, S/N ratio ~900). An important advantage of CellFluor™ GST is high cell-permeability and this probe can measure intracellular GST activity by green fluorescent intensity under live cell condition. As the DNB group is a well-characterized substrate for various types of GST members, CellFluor™ GST is able to monitor pan-GST activity at least including 6 cytosolic GST subfamilies and MGST family. As only addition of CellFluor™ GST to cell culture medium is required to monitor intracellular GST activity, flexible applications are available. CellFluor™ GST is a powerful tool not only to investigate physiological GST activities in live cell upon any biological stimulation, but also to develop GST inhibitors under live cell condition.
Comparison between CellFluor™ GST and Conventional CDNB
|
Probe
|
CellFluor™ GST
|
CDNB
|
||
|
Product Code
|
-
|
|||
|
Colorimetric or Fluorescence
|
Green fluorescence
|
UV
|
||
|
Wavelength
|
Ex 496 nm/Em 520 nm
|
~340 nm
|
||
|
Target
|
Wide range of GST family members
|
|||
|
Application
|
in vitro assay
(Lysate or purified enzymes etc.)
|
Possible
|
Possible
|
|
|
Live cells
|
Fluorescent plate reader
|
Possible
|
Impossible
|
|
|
Fluorescent imaging
|
Possible
|
Impossible
|
||
|
Flow cytometry
|
Possible
|
Impossible
|
||
|
Sensitivity
|
High
(Fluorescent emission)
|
Low
(UV absorbance)
|
||
|
High throughput & easy protocol
|
High
(Compatible in live cell and no-wash requirement)
|
Low
(Lysate preparation required)
|
||
|
Multimodality
|
Possible
(Ex. blue and red fluorescent dyes are compatible)
|
Difficult
|
||
Principle
CellFluor™ GST is a modified Rhodamine 110, a famous green dye, protected with two 2,4-dinitrobenezensulfonamides (DNBs) which is a typical GST substrate and is extremely quenched (quantum yield = 0.0007). When DNBs groups are removed by GSTs for converting a GS-conjugate, the free-Rhodamine 110 dye (quantum yield = 0.647) is released and emits strong green fluorescence (Ex. 496 nm/ Em 520 nm).
CellFluor™ GST (bis-DNs-Rh or DNs-Rh in the original papers) has been originally developed as free-thiol detection reagent but the later studies shows this probe can also detect GST activity. Reactivity of CellFluor™ GST to GSTs is much higher than a reactivity to free-thiol, now CellFluor™ GST is a powerful probe for intracellular GST activity under live cell condition. Reactivities for cytosolic GSTs (GSTA, GSTM, GSTP, GSTO, GSTT, GSTZ) and microsomal GST (MGST) were experimentally confirmed.
※ Note : CellFluor™ GST can react weakly with free-thiol containing compounds such as GSH, DTT etc. Free-thiol containing compounds in assay buffer may interfere CellFluor™ GST assay. If assay buffers contain free-thiol compounds, setting of appropriate control experiment is highly recommended.
In the cell-based assay, Rhodamine 110 dye converted by intracellular GSTs diffuses intracellularly, mainly into mitochondria, but is not highly intracellularly retained and is gradually released into the medium. For this reason, in fluorescence plate reader experiments, we recommend to measure the fluorescence intensity without exchanging the medium after adding CellFluor™ GST. On the other hand, in fluorescent imaging experiments, free-Rhodamine 110 released into the media may become a background signal during observation, and immediate observation after washing is recommended.
Features
- GST enzymatic activity induces release of free-Rhodamine 110 dye and emission of green fluorescence (Ex 496 nm/Em 520 nm).
- Compatible with both cell-based assays (fluorescent plate reader, fluorescent imaging, flow cytometry) and in vitro assays (purified GST enzymes, cell or tissue lysates etc.).
- For in vitro assay, this probe has higher sensitivity than conventional CDNB.
- In live cell assays, this probe can be used simply by adding it to the culture medium due to its cell membrane permeability. It also exhibits a high signal-to-noise ratio (quantum yield ratio ~900-fold) before and after the GST response, allowing measurements without the need for washing procedures.
- This probe can react with wide range of GST family members.
Validated GST Subfamily
- GSTα (GSTA1, GSTA2, GSTA3, GSTA4) GSTμ (GSTM1, GSTM2) GSTπ (GSTP1) GSTω (GSTO1) GSTθ (GSTT1) GSTζ (GSTZ1) MGST (MGST1)
※ Analysis using purified proteins of recombinant GSTO1 and GSTT1 shows that GSTO1 and GSTT1 are less active for CellFluor™ GST than other cytosolic GSTs (GSTA1, GSTM1, GSTP1, GSTZ1).
Spectrum
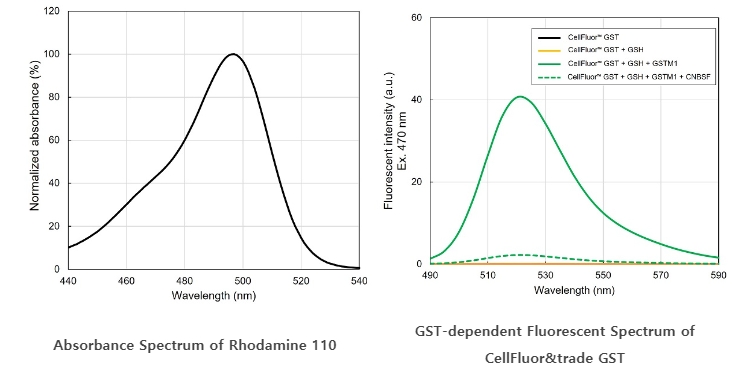
- Left : Normalized absorbance spectrum of Rhodamine 110. Maximum absorbance ~495 nm.
- Right : Fluorescent spectrum of CellFluor™ GST (1 μM) with or without GSH, recombinant GSTM1, and ☞ CNBSF, a GST inhibitor, excited at 470 nm in assay buffer (50 mM sodium phosphate (pH 7.4), 150 mM NaCl) was measured. While CellFluor™ GST only and in the presence of 10 μM GSH showed little fluorescent intensity, GSTM1 clearly increased fluorescent intensity (maximum ~525 nm). When GSTM1 was pre-incubated with GSH and ☞ CNBSF (a GST irreversible inhibitor), fluorescent intensity was dramatically suppressed.
Application data
Kinetic Measurement of GST Activity in vitro
Fluorescent intensity (Ex 470 nm/Em 525 nm) in the presence and absence of human recombinant GSTM1 (0.2 μg/ml) in assay buffer (50 mM sodium phosphate (pH 7.4), 150 mM NaCl) containing 5 μM CellFluor™ GST and 10 μM GSH was measured over time for 30 min. Whereas slow fluorescence increased was observed under the condition without GSTM1, in the presence of GSTM1 fluorescent intensity was remarkably increased and a linear increase in signal was observed in the 30 min range.
※ The other cytosolic GST submembers including GSTA1, GSTP1, GSTO1, GSTT1, GSTZ1 showed similar results.
※ As CellFluor™ GST could react with thiol group weakly (please refer to ☞ Principle). In in vitro assay, CellFluor™ GST assay needs reduced GSH as a cofactor of GST. High concentration of reduced GSH may be background signal of CellFluor™ GST probe. Please empirically optimize concentration of GSH and setting of appropriate negative control experiments is highly recommended.
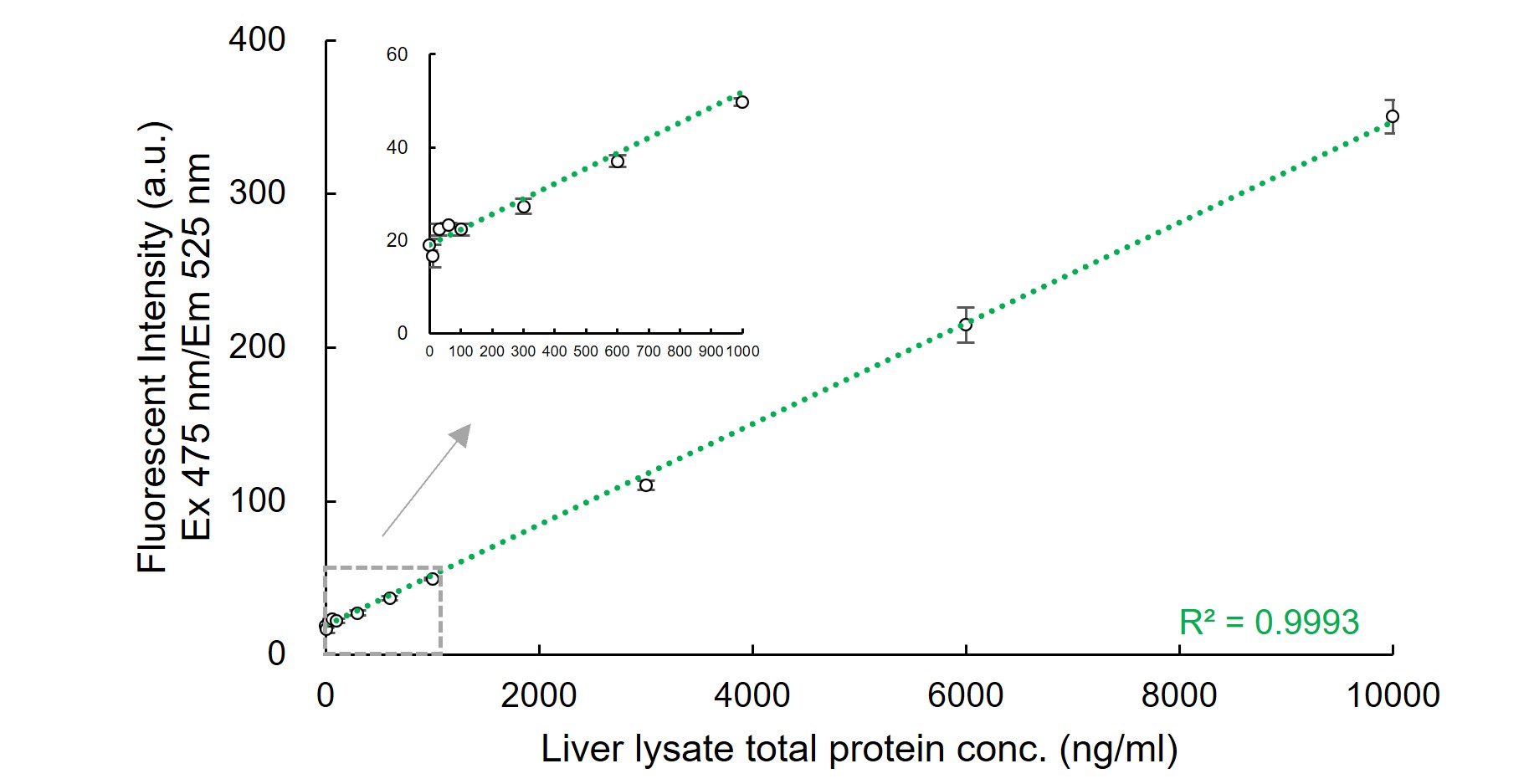
Dose-dependent Fluorescent Response of CellFluor™ GST using Liver Lysate
Mouse liver lysate was added to assay buffer (50 mM sodium phosphate (pH 7.4), 150 mM NaCl, 1% NP-40, 100 μM GSH) to be a total protein concentration of 10-10,000 ng/ml. CellFluor™ GST (final conc. 1 μM) was added to them and incubated for 30 min at RT. Fluorescent intensity (Ex 475±5 nm/Em 525±10 nm) was measured by a fluorescent plate reader. A total protein dose-dependent fluorescence response was confirmed with high correlation coefficient (R2=0.9993). The detection limit in this experiment was estimated to be around 100 ng/ml liver total protein.
※ When a conventional CDNB assay (GSH 1 mM, CDNB 1 mM) was performed using the same liver samples. Then, the detection limit was around 1000 ng/ml.
Cell Number-dependent Fluorescent Response of CellFluor™ GST in Cell-based Assay
Cell number-dependent fluorescent response of CellFluor™ GST in cell-based assay CHO cells were seeded in 96 well plate in 2x dilution series from 2×105 cells/well and cultured in DMEM containing 10% FBS for 4 hours. After cell adhesion was confirmed, the culture media were replaced with serum- and phenol red-free DMEM containing 2 μM CellFluor™ GST. Fluorescent intensities were measured every hour with a fluorescent plate reader (Ex 475±5 nm/Em 525±10 nm). Time-dependency of fluorescent response was observed, and fluorescent intensity was highly correlated with cell number at all reaction times. The detection limit was estimated to be about 300 cell/well in this experiment.
Cell-based Comparison of GST Activities
Four cell line (Chinese hamster ovarian cancer cell line CHO, human renal cancer cell line 786-O, mouse neuroblastoma cell line Neuro2a, and human fetal kidney cell line HEK293) were seeded in 96 well plate at 1×104 cell/well and cultured in DMEM containing 10% FBS for 20 hours. Then, the medium was replaced with serum-free and phenol red-free DMEM containing 2 μM CellFluor™ GST and cells were incubated for 1 hour. Fluorescent intensities (Ex 475±5 nm/Em 525 nm±10 nm) was measured with a fluorescence plate reader without medium exchange. Among the four cell lines tested here, CHO cells showed the highest GST activities.
Cell-based Assay for Monitoring Drug-induced Activity Change of GST
786-O, Neuro2a and CHO cells were seeded in 96 well plates at 1×104 cells/well, and cultured in DMEM containing 10% FBS for 4 hours. Then media were replaced to serum-free DMEM, and cells were cultured in the presence of retinoic acid (5 μM) or Tunicamycin (1 μg/ml) for 20 hours. Only ☞ CNBSF (100 μM), a potent irreversible GST inhibitor, was incubated in HBS for 1 hour. The medium was replaced with HBS, CellFluor™ GST (2 μM) was added, and cells were incubated for 1 hour. Then fluorescence intensity was measured with a fluorescent plate reader (Ex 475±5 nm/ Em 525±10 nm) without medium change. In all cell lines, cellular GST activity was markedly suppressed by the addition of ☞ CNBSF. Retinoic acid increased GST activity and tunicamycin decreased GST activity.
Fluorescent Live Cell Imaging
Three cell lines (CHO, 786-O, and Neuro2a) were seeded into glass bottom dishes and culture for 20 hours in 10% FBS-containing DMEM. Culture medium was replaced to serum- and phenol red-free DMEM and CellFluor™ GST (final 30 μM) was incubated for 10 min. Just before observation, the culture media were replaced to fresh serum- and phenol red-free DMEM and quickly observed by epifluorescent microscopy (Ex 435-475 nm/Em 530-543 nm). In all cell lines, green fluorescent signals were observed from inside of the cells. On the other hand, when 100 μM ☞ CNBSF, a potent irreversible GST inhibitor, was pretreated to cells for 30 min, the fluorescent signals were dramatically suppressed.
In vitro Assay for Comparison of Tissue GST Activities
Four mouse tissues (liver, brain, spleen, and kidney) were cut into small pieces, lysed in lysis buffer (50 mM sodium phosphate (pH 7.4), 150 mM NaCl, 1% NP-40), and centrifuged to obtain soluble fraction. The total protein concentration of the soluble fraction was determined by the BCA assay and prepared to 1 mg/ml for each tissue. Each tissue lysate was added to assay buffer (50 mM sodium phosphate (pH 7.4), 150 mM NaCl, 1% NP-40, 100 μM GSH) to a final concentration of 0.1 mg/ml of total protein. After 30 min reaction at RT, fluorescence intensity was measured with a fluorescence plate reader (Ex 475±5 nm/Em 525±10 nm). The samples were also pretreated with ☞ CNBSF (100 μM final concentration) prior to the addition of CellFluor™ GST. In the four tissues tested, significantly stronger GST activity was observed in the liver. In all tissues, the activity was inhibited by ☞ CNBSF.
Reference
- Shibata, A., et al., "Rhodamine-based fluorogenic probe for imaging biological thiol.", Bioorg. Med. Chem. Lett., 18(7), 2246~2249(2008). [PMID:18358719]
- Alander, J., et al., "Characterization of a new fluorogenic substrate for microsomal glutathione transferase 1.", Anal. Biochem., 390(1), 52~56(2009). [PMID:19348782]
- Zhang, J., et al., "Synthesis and characterization of a series of highly fluorogenic substrates for glutathione transferases, a general stategy.", J. Am. Chem. Soc., 133(35), 14109~14119(2011). [PMID:21786801]
- Shishido, Y., et al., "A covalent inhibitor for Glutathione S-Transferase Pi (GSTP1-1) in Human Cells.", ChemBioChem., 20(7), 900~905(2019). [PMID:30548113]

코아사이언스 coresciences Funakoshi Inc. Japan 후나코시 한국 대리점 세포 기반 글루타티온 S-전달효소 활성 측정 프로브 분석 글루타티온 S-전달효소 활성 분석, 연구, 측정 프로브
'실험.연구용 추천상품' 카테고리의 다른 글
- Total
- Today
- Yesterday
- matrix-driven delivery pellet
- 콘드로이틴 황산 올리고당
- 미니멸균백
- 고수용성 콘드로이틴
- 오토클레이브백
- 면역화학분석
- 바이오헤저드백
- time release pellets
- solar cells
- 바이오하자드백
- 건스터바이오텍
- 파라핀 블럭
- 연속절편
- gradient gel
- 조직절편제작
- OPV
- 후나코시
- 조직염색절편제작
- material science
- OLED
- allview PAGE buffer
- Funakoshi
- 코아사이언스
- 형광염색
- Sterlitech
- 조직절편
- 파라핀 블록
- Coresciences
- filter
- 막필터
| 일 | 월 | 화 | 수 | 목 | 금 | 토 |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| 27 | 28 | 29 | 30 |