티스토리 뷰
DPP-DTT; PDPP2T-TT-OD [M0311A7][CAS no. 1260685-66-2 (1444870-74-9)]_Ossila - 코아사이언스
코피디 2023. 2. 28. 15:48안녕하세요!
Material Science 전문제조사 Ossila Ltd 수입/공급 업체 코아사이언스 입니다.
자세한 상품 정보 및 관련 문의는 전화 02-858-0328 또는 info@coresciences.co.kr로 문의주시기 바랍니다.
감사합니다!
DPP-DTT
=PDPP2T-TT-OD
Cat.# M0311A7 / Size: 100mg, 250mg, 500mg, 1g, 2g
*Cat.# 및 세부사양은 제조사 Lot.# 재고 상황에 따라 수시 변동 될 수 있습니다.
DPP-DTT, high quality and high purity semiconducting polymer
High performance p-type polymer and donor material for BHJ photovoltaics
DPP-DTT from Ossila was used in the high-impact paper (IF 18.81), Stretchable Mesh-Patterned Organic Semiconducting Thin Films on Creased Elastomeric Substrates, S. Kim et al., Adv. Funct. Mater., 2010870 (2021); DOI: 10.1002/adfm.202010870.
Luminosyn™ DPP-DTT (also referred to as PDPP2T-TT-OD) is now available featuring:
- High molecular weight - higher molecular weight offers higher charge mobility
- High purity - DPP-DTT is purified via Soxhlet extraction with methanol, hexane and chlorobenzene under an argon atmosphere
- Batch-specific GPC data - so you have confidence in what you are ordering. Also, GPC data is always convenient for your thesis and publications
- Large quantity orders - so you can plan your experiments with polymer from the same batch
General Information
|
CAS number
|
1260685-66-2 (1444870-74-9)
|
|
Chemical formula
|
(C60H88N2O2S4)n
|
|
HOMO / LUMO
|
HOMO = -5.2 eV, LUMO = -3.5 eV [2]
|
|
Synonyms
|
|
|
Solubility
|
Chloroform, chlorobenzene and dichlorobenzene
|
|
Classification / Family
|
Bithiophene, Thienothiophene, Organic semiconducting materials, Low band-gap polymers, Organic photovoltaics, Polymer solar cells, OFETs
|

Chemical structure and product image of DPP-DTT, CAS No. 1260685-66-2.
OFET and Sensing Applications
The exceptional high mobility of this polymer of up to 10 cm2/Vs [2] via solution-processed techniques, combined with its intrinsic air stability (even during annealing) has made PDPP2T-TT-OD of significant interest for OFET and sensing purposes.
While the highest mobilities require exceptional molecular weights of around 500 kD (and with commensurate solubility issues), high mobilities in the region of 1-3 cm2/Vs can still be achieved with good solution-processing at around 250 kD. As such, we have made a range of molecular weights available to allow for different processing techniques.
In our own tests, we have found that by using simple spin-coating onto an OTS-treated silicon substrate (using our prefabricated test chips), high mobilities comparable to the literature can be achieved (1-3 cm2/Vs). Further improvements may also be possible with more advanced strain-inducing deposition techniques.
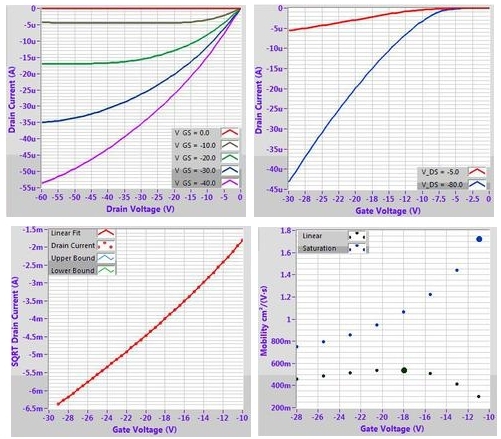
Example OFET characteristics for DPP-DTT (M313) solution processed from chlorobenzene on a 300 nm SiO2 substrate treated with OTS. Output characteristic (top left), transfer curves (top right), mobility fitting (bottom left) and calculated mobility (bottom right).
Photovoltaic Applications
Although shown as a promising hole-mobility polymer for OFETs, when used as the donor material in a bulk heterojunction photovoltaic (with PC70BM as the acceptor), initial efficiencies of 1.6% were achieved for DPP-DTT [3]. The low device metrics were attributed to poor film morphology. However, a higher efficiency of 6.9% was achieved by using thicker film (220 nm) [4].
PDPP2T-TT-OD has also recently been used successfully as an active-layer dopant material in PTB7-based devices [5]. An improvement in device performance was observed, with average efficiencies increasing from 7.6% to 8.3% when the dopant concentration of DPP-DTT was 1 wt%. The use of DPP-DTT as a high-mobility hole-interface layer for perovskite hybrid devices has also been investigated [6].
Synthetic route
DPP-DTT synthesis: DPP-DTT was synthesised by following the procedures described in [2] and [3] (please refer to the following references):
With 2-thiophenecarbonitrile and dimethyl succinate as starting materials in t-amyl alcohol, it gave 3,6-Dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione. Alkylation of 3,6-Dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione with 2-octyldodecylbromide in dimethylformamide afforded 3,6-bis(thiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione. Further bromination gave 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (M1).
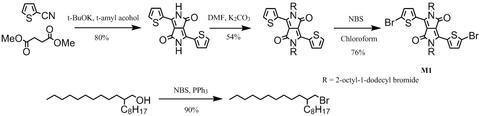
Further reaction of M1 with 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene (M2) under Stille coupling conditions gave the target polymer DPP-DTT, which was further purified via Soxhlet extraction with methanol, hexane and then chloroform.

Batch information
|
Batch*
|
Mw
|
Mn
|
PDI
|
Stock info
|
|
M0311A4
|
152,923
|
55,143
|
2.77
|
Discontinued
|
|
M0311A5
|
100,105
|
39,080
|
2.56
|
Discontinued
|
|
M0311A6
|
87,278
|
37,778
|
2.31
|
Discontinued
|
|
M0311A7
|
111,029
|
45,803
|
2.42
|
In Stock
|
References:
1. A High Mobility P-Type DPP-Thieno[3,2-b]thiophene Copolymer for Organic Thin-Film Transistors, Y. Li et al., Adv. Mater., 22, 4862-4866 (2010)
2. A stable solution-processed polymer semiconductor with record high-mobility for printed transistors, J. Li et al., Nature Scientific Reports, 2, 754, DOI: 10.1038/srep00754 (2012)
3. Synthesis of low bandgap polymer based on 3,6-dithien-2-yl-2,5-dialkylpyrrolo[3,4-c]pyrrole-1,4-dione for photovoltaic applications, G. Zhang et al., Sol. Energ. Mat. Sol. C., 95, 1168-1173 (2011)
4. Efficient small bandgap polymer solar cells with high fill factors for 300 nm thick films, Li W et al., Adv Mater., 25(23):3182-3186 (2013); doi:10.1002/adma.201300017.
5. Enhanced efficiency of polymer solar cells by adding a high-mobility conjugated polymer, S. Liu et al., Energy Environ. Sci., 8, 1463-1470 (2015)
6. Electro-optics of perovskite solar cells, Q. Lin et al., Nature Photonics, 9, 106-112 (2015)
7. A Vertical Organic Transistor Architecture for Fast Nonvolatile Memory, X. She et al., adv. Mater., 29, 1604769 (2017); DOI: 10.1002/adma.201604769.
8. Solvent-Free Processable and Photo-Patternable Hybrid Gate Dielectric for Flexible Top-Gate Organic Field-Effect Transistors, J. S. Kwon et al., ACS Appl. Mater. Interfaces, 9 (6), 5366–5374 (2017); DOI: 10.1021/acsami.6b14500.
관련 상품
코아사이언스 coresciences Ossila Ltd UK 영국 한국 Korea distributor Top OFET Products Top OLED Products Top OPV Products Nanotubes Graphene Perovskite Product 페로브스카이트 Organic Electronics Semiconducting Polymers 반도체 중합체 반도체 고분자 Luminosyn OLED, OPV, Perovskite, OFET Sensing Graphene 그래핀
'Material Science' 카테고리의 다른 글
- Total
- Today
- Yesterday
- 파라핀 블럭
- 면역화학분석
- gradient gel
- 콘드로이틴 황산 올리고당
- OLED
- 건스터바이오텍
- 연속절편
- OPV
- 파라핀 블록
- matrix-driven delivery pellet
- filter
- 후나코시
- Sterlitech
- 바이오헤저드백
- 조직절편제작
- allview PAGE buffer
- 바이오하자드백
- time release pellets
- material science
- 형광염색
- 조직절편
- 조직염색절편제작
- Funakoshi
- Coresciences
- 오토클레이브백
- 미니멸균백
- 고수용성 콘드로이틴
- 막필터
- solar cells
- 코아사이언스
| 일 | 월 | 화 | 수 | 목 | 금 | 토 |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| 27 | 28 | 29 | 30 | 31 |




