티스토리 뷰
[콜라겐 변성 확인 분석/변성된 콜라겐 검출/연구 시약] BindCOL, biotin-conjugated, Denatured Collagen Detection Reagent [FDV-0035]_Funakoshi - 코아사이언스
코피디 2020. 11. 10. 10:50BindCOL, biotin-conjugated, Denatured Collagen Detection Reagent
Cat.# FDV-0035 / Size: 60 ug
각종 병리 상태의 마커(marker)로 주목받고 있는 변성 콜라겐(denatured collagen)을 고감도로 간편하게 확인할 수 있는 연구용 시약 입니다. 항체와는 달리, 변성된 콜라겐을 특이적으로 검출 할 수 있기 때문에 콜라겐의 생리 · 병리학 연구에 유용합니다. 본 시약은 세포 내 생합성 과정에 있는 1가닥 상태의 콜라겐 검출에도 응용할 수 있습니다.
Background
Collagen is the most abundant component of the extracellular matrix (ECM) and constitutes a large protein family in mammals. Collagen family shares unique triple-helical structures (Figure 1, left) consisting of three polypeptides that contain frequent repeats of Gly-Pro-Hyp (4-hydroxyproline) triplets. In human, 27 types are identified as the collagen family. Among them, types I, II and III occupy the majority (~90%) of the family in the body.
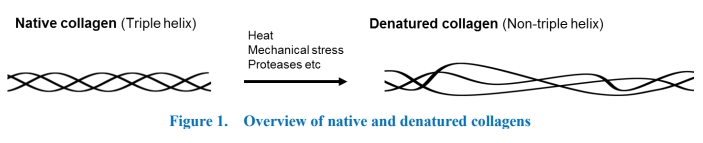
Collagens have various functions from regulation of cellular activities, such as cell adhesion, migration and invasion, to mechanical support of tissues. In these processes, the dynamic remodeling of collagens including de novo production and degradation/denaturation is tightly regulated. Abnormal regulations of collagen-remodeling derived from excess production of unfolded or misfolded collagens or highly degradation of collagens leads to pathological conditions. Fibrosis in liver, osteoarthritis in cartilage, atherosclerosis in blood vessels and abnormal invasion of cancer cells are well-known examples of imbalance of collagen remodeling. Recent evidences indicate the increased amount of denatured collagen is associated with these diseases. Denatured collagen is the loss of triple helical structure induced by heat, proteases, mechanical stress, and chemical modification (Figure 1, right). Once triple helical structures are destroyed, each polypeptide cannot reorganize the triple-helical structure. As denatured collagen is one of the pathological markers, detecting method for denatured collagens will be a good tool for pathophysiological research. Antibodies are one of the general tools for collagen research but cannot distinguish between native and denatured collagens. Only few antibodies are established to specifically detect denatured collagen, but they recognize only one specific collagen isoform. The detection probes satisfying following three points are highly desired. 1) Denatured collagen specific, 2) highly sensitive, and 3) broad cross-reactivity for various types of collagens. Recently, chemically synthesized collagen mimetic peptides (CMP) containing collagen-like amino acid sequences (Gly-Pro-Hyp)n are applied in detection of denatured collagens (Figure 2). CMPs selectively hybridize in the unfolded region of collagens. However, single-stranded CMPs (ssCMP) are preferentially self-assembled to homo trimer in water and it dramatically reduces binding affinity to denatured collagens. To avoid self-assembly of ssCMPs a pre-heating step is necessary. Pre-heating promotes dissociation of self-assembled trimers and increases the binding affinity of ssCMPs to denatured collagens. The experimental pre-heating process gives not only researcher’s increased labor but also experimental bias in each trial, ssCMPs are not sufficient tools for detecting various denatured collagens. To overcome this problem, Dr. Takaki Koide, Waseda University, developed the next generation denatured collagen detecting peptide. The novel peptide has highly unique structure which is a strained cyclic CMP (scCMP) and has optimized anionic charges. Due to strained cyclic structure and anionic charges, the self-assembly of the peptides are suppressed. The scCMP improves not only pre-heating step but also binding affinity for denatured collagen, over 10-fold higher than ssCMP. Our “Denatured Collagen Detecting Reagent” (scCMP) shows dramatically higher sensitivity than ssCMP without preheating step.

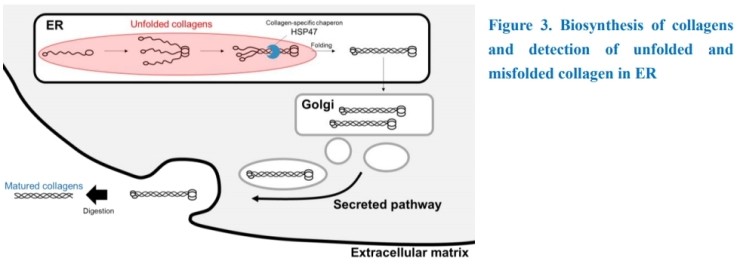
Collagen biosynthesis is another important process of dynamic collagen remodeling. Collagen polypeptides are firstly synthesized and post-translationally modified by prolyl hydroxylase in endoplasmic reticulum (ER). Three polypeptide chains cannot an assemble triple helical structure by itself. Collagen-specific molecular chaperon, HSP47 promotes formation of trimers. Folded collagens are transferred to Golgi apparatus and secretory pathway. Abnormal collagen synthesis, maturation or secretory also induces some diseases. Our “Denatured Collagen Detecting Reagent” is applicable detecting reagent for unfolded or misfolded collagens in ER under the biosynthetic pathway.
Description
Molecular weight : ~4,700 Da
Probe structure : Figure 4
Solubility : Soluble in water
Label : Biotin

Applications
- Detection of denatured collagen
- Detection of intracellular unfolded and misfolded collagen
- Detection of total collagen in Western Blotting system
Storage
Store powder at -20oC. Please avoid humid conditions
Reference data
Quantitative analysis of binding affinity of both ssCMP and “Denatured Collagen Detection Reagent”
Wells on 96 well plate were pre-coated with heat-denatured collagen I. The wells were washed by ELISA buffer (20 mM HEPES-Na (pH 7.5), 100 mM NaCl, and 0.005% Tween-20) three times and blocked by 0.5% skim milk. After washing the wells, ssCMP or “Denatured Collagen Detection Reagent”-containing solution were added into the wells and incubated for 1 hour. The wells were washed by ELISA buffer three times and probed with streptavidin-HRP conjugated. Binding intensity was estimated with a colorimetric substrate for HRP

* Research use only, not for human or animal therapeutic or diagnostic use !
References
1. Takita, K. K., Fujii, K. K., Ishii, K., Koide, T., Org. Biomol. Chem., 17, 7380-7387 (2019) Structural optimization of cyclic peptides that efficiently detect denatured collagen.
2. Takita, K. K., Fujii, K. K. Kadonosono, T., Masuda, R., Koide, T., ChemBioChem., 19, 1613-1617 (2018) Cyclic Peptides for Efficient Detection of Collagen.
관련 상품
| Cat.# | Product | Target | Application |
| FDV-0023 | Anti-Laminin alpha3B, Human, Mouse-Mono (F7) | Laminin alpha3B | IHC, WB, IP, ELISA |
| FDV-0024 | Anti-Laminin alpha3A, Human, Mouse-Mono (BG5) | Laminin alpha3A | IHC, WB, IP, ELISA |
| FDV-0025 | Anti-Laminin gamma2 N-terminal fragment, Human, Mouse-Mono (P2H) | Laminin gamma2 N-terminal fragment |
IHC, WB, ELISA |
| FDV-0026 | Anti-Laminin 511, Human, Mouse-Mono (12D) | Trimeric Lm511 structure | IHC, WB, IP, ELISA |

코아사이언스 coresciences Funakoshi Inc. Japan 후나코시 한국 대리점 콜라겐 변성 변성된 콜라겐 병리학 질병 질환 마커 표지자 marker denatured collagen
'연구용 시약' 카테고리의 다른 글
- Total
- Today
- Yesterday
- 건스터바이오텍
- OPV
- gradient gel
- allview PAGE buffer
- material science
- 콘드로이틴 황산 올리고당
- 오토클레이브백
- 후나코시
- 조직염색절편제작
- OLED
- 코아사이언스
- 막필터
- filter
- 파라핀 블록
- Funakoshi
- time release pellets
- 조직절편
- Sterlitech
- 파라핀 블럭
- 바이오하자드백
- 면역화학분석
- 연속절편
- solar cells
- 바이오헤저드백
- 고수용성 콘드로이틴
- Coresciences
- 형광염색
- 조직절편제작
- matrix-driven delivery pellet
- 미니멸균백
| 일 | 월 | 화 | 수 | 목 | 금 | 토 |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 25 | 26 | 27 | 28 | 29 | 30 | 31 |
