티스토리 뷰
ER-Protein Capture Kit
Cat.# FDV-0039 / Size: 1 kit
본 상품은 ER 단백질 기능 연구를 위해 ER 관련 단백질들을 특이적으로 분리하는 키트 입니다.
본 키트에서 사용되는 ERM 분자는 ER 막에 대해 높은 친화도를 지녀 ER 내로 특이적으로 빠르게 축적될 수 있습니다.
ER 내로 침투한 ERM은 다양한 ER 단백질들과 공유결합을 형성하며 표지됩니다.
그런 다음 세포를 용해시켜 이들 표지된 ER 단백질들을 immunoaffinity purification 방법을 이용하여 선택적으로 분리합니다.
분리된 표지 ER 단백질들은 SDS-PAGE, LS/MS 기반 프로테오믹스 또는 Western blot 방법으로 분석할 수 있습니다.
본 키트를 이용하면 ER 연관 단백질들을 초원심분리기(Ultracentrifuge)와 같은 특수 장비 없이 쉽게 분리할 수 있습니다!
다양한 조건하에서 ER의 여러 기능을 본 키트로 선택적으로 분리한 단백질들을 통해 탐구할 수 있습니다.
Product Background
Endoplasmic reticulum (ER) is the largest organelle in the cell and has unique and dynamic tubular or sheet structures. ER plays essential roles in biosynthesis, precise folding and quality check of proteins and is a traffic origin of secreted pathway proteins including the Golgi apparatus, exocytosis, plasma membrane, and extracellular proteins. The major functions of ER are not only protein synthesis, but also carbohydrate metabolism, calcium storage, lipid metabolism, and lipid droplet synthesis. Although important roles of ER, biochemical isolation methods are highly limited presently because of its complicated structures. Some biochemical approaches with ultracentrifugation are utilized to roughly isolate ER membrane fractions, but conventional methods require time-consuming procedures with specialized equipment and show frequent contamination of other organelles such as endosomes, etc. To access the function of ER proteins, an isolation method for ER-associated proteinsspecifically with an easy procedure should be expected.
Our “ER-Protein Capture Kit” is based on the ER-localizable Reactive Molecule (ERM) technology originally developed by Dr. Itaru Hamachi and Dr. Tomonori Tamura, Kyoto University (Ref. 1). In this kit, there are two components, ERM (component A) and anti-rhodol antibody (component B). An overview of the kit principle is shown in Figure 1. ERM is a small compound which has two units, a rhodol-type green fluorescent dye, and a thioester-type protein labeling group (Figure 1). The rhodol-type dye has a high affinity to ER membranes and specifically and quickly accumulates into ER (Step-1 left). ER-specificity of the rhodol-type dye is comparable to a conventional ER staining reagent, fluorescent-labeled Glibenclamide. Immediately after the addition and specific accumulation of ERM into the ER, the labeling group of ERM reacts with nucleophilic amino acids in ER-proteins forming a covalent bond to proteins to label the rhodol-tag (Step-1 right). Subsequently, cells are lysed by cell lysis buffers (Step-2), and rhodol-tagged proteins are selectively purified by immunoaffinity purification with anti-rhodol antibody (Step-3). Purified rhodol-tagged proteins are separated by SDS-PAGE (Step-4) and analyzed by LS/MSbased proteomics or western blot method (Step-5). This kit enables to purify ER-associated proteins with easy procedures and no special equipment such as ultracentrifuge machine. Ref.1 shows proteomic analysis of ERassociated proteins using ERM. ERM-based proteomics shows that ERM enables to identify not only ER-resident proteins but also secreted pathway proteins including Golgi apparatus, plasma membrane and extracellular protein which are recruited to ER before their final destination. Ref.1 also shows a quantitative analysis of ER-associated proteins during tunicamycin-induced ER-stress and revealed some proteins clearly increased or decreased under the ER-stress condition. “ER-Protein Capture Kit” is a powerful tool to selectively purify ER-associated proteins to evaluate various ER roles.

Description

<Note> ERM can be purchased separately (Catalog #FDV-0029, product name ERseeing).
Application
- ER-associated proteins specific fluorescent labeling
- ER-associated proteins selective purification
- Comprehensive identification of ER-associated proteins by LC/MS proteomics
- Quantitative analysis of ER-associated proteins by quantitative MS
- Individual detection of ER-associated proteins by western blotting
Reference Data
Absorption and fluorescent spectrum of ERM
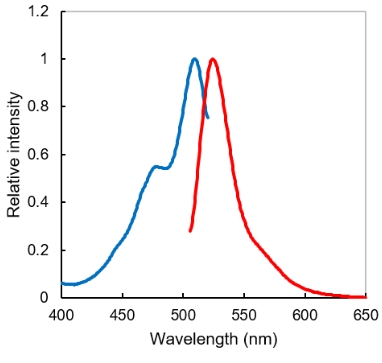
Excitation (blue) and fluorescent (red) spectrum. Exmax/Emmax = 509/524 nm. Commercial FITC filter sets are compatible.
ER specificity

HeLa cells were stained with ERseeing (100 nM) and organelle markers, Glibencramide-type ER staining, lysosomal staining, mitochondrial staining, and Golgi apparatus staining. ERseeing was highly overlapped with conventional Glibencramide-type ER staining (Piason coefficiency >0.9) but not correlated with lysosome marker or mitochondria marker. Only a small portion of staining by ERseeing was overlapped with Golgi apparatus staining. It was considered that this is attributed to the vesicle transport of ERseeing or ERseeing labeling proteins from ER to Golgi apparatus. The ER-to-Golgi trafficking inhibitor decreased overlap between ERseeing-staining and the Golgi apparatus-staining (Detail information is described in Ref. 1).
Application Data
Comprehensive identification of ER-associated proteins by LC/MS-proteomics
HeLa cells in 10 cm dish were treated with 100 nM ERM for 1 hour and lysed by cell lysis buffer. After total protein precipitation by chilled acetone, proteins were resolved in 4% SDS lysis buffer (25 mM Tris (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 4% SDS, 1% sodium deoxycholate) with sonication, and then diluted 4-fold with RIPA buffer to ~1% SDS concentration. Total protein concentration was measured with BCA assay and 2.5 mg of total proteins were applied for immunoaffinity purification. Protein A-beads and anti-rhodol antibody complex was added to total protein solution and incubate for overnight at 4oC. After incubation, the beads were washed and purified proteins were eluted with SDS-PAGE loading buffer. Proteins were separated by SDS-PAGE and the gel was fixed. After slicing the gel, sliced gels were subjected to in-gel digestion using trypsin/Lys-C. The eluted peptides derived from the gel were purified and subjected to nanoLC-MS/MS analysis.
Total of 146 proteins were identified in this experiment. 63 proteins were categorized in ER-resident proteins and 73 proteins were categorized in the secretory pathways such as membrane, Golgi apparatus, and extracellular proteins. As secretory pathway proteins basically move to the final destination via ER, ERM-based assay could identify ER-associated proteins with around 93% probability.
Note: ER proteins (63 proteins) were assigned by two methods. One is based on database analysis (28 proteins) and another is manually assigned with a literature survey (35 proteins). The information for ER-associated proteins in the commercially available database is still incomplete. Please note data analysis of identified proteins should be carefully performed. The detail annotation method was described in Ref.1.

Quantitative profiling of ER-associated proteins during ER stress by SILAC assay
HeLa cells were continuously grown in “light” SILAC media or “heavy” SILAC media. The “heavy” isotopelabeled cells and the “light” isotope-labeled cells were treated with tunicamycin (2.5 g/ml), a chemical inducer of ER-stress, and DMSO as vehicle control for 4 hours, respectively. After tunicamycin or DMSO treatment, both cells were incubated with 100 nM ERM for 1 hour and lysed by cell lysis buffer. Equal amounts of “heavy” isotope-labeled proteins and “light” isotope-labeled proteins were mixed in a 1:1 ratio and rhodol-labeled proteins were purified with anti-rhodol antibody/protein A-beads and analyzed described above. A total of 87 proteins were identified and quantified. Among 87 proteins, 39 proteins (45%) were classified as ER proteins and 84 proteins (97%) were assigned with ER-associated proteins including secretory pathway proteins. SILAC analysis indicates 6 proteins were upregulated by more than 2-fold in tunicamycin-treated cells. On the other hand, 7 proteins were downregulated by more than 2-fold in tunicamycin-treated cells.

* Research use only, not for human or animal therapeutic or diagnostic use !
References
1. Fujisawa et al., J. Am. Chem. Soc., 140, 17060-17070 (2018) Chemical Profiling of the Endoplasmic Reticulum Proteome Using Designer Labeling Reagents.

코아사이언스 coresciences Funakoshi Inc. Japan 후나코시 한국 대리점 형광염색 시약 세포내 소기관 ER 소포체 관찰 이미징 이미지 구조 관찰 기능 연구 세포내 소기관 소포체 연관 단백질 ER 상주 단백질 분리 정제 분석
'연구용 시약' 카테고리의 다른 글
- Total
- Today
- Yesterday
- Sterlitech
- 오토클레이브백
- 바이오하자드백
- Funakoshi
- solar cells
- OPV
- time release pellets
- 조직염색절편제작
- 막필터
- 형광염색
- 건스터바이오텍
- 미니멸균백
- OLED
- allview PAGE buffer
- matrix-driven delivery pellet
- 콘드로이틴 황산 올리고당
- Coresciences
- 고수용성 콘드로이틴
- 코아사이언스
- 파라핀 블럭
- 파라핀 블록
- 후나코시
- gradient gel
- 바이오헤저드백
- 연속절편
- filter
- 면역화학분석
- 조직절편제작
- 조직절편
- material science
| 일 | 월 | 화 | 수 | 목 | 금 | 토 |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| 29 | 30 |
