티스토리 뷰
Azithromycin EP Impurity C [VL186009][CAS No. 620169-47-3]_Veeprho - 코아사이언스
코피디 2024. 1. 22. 13:55안녕하세요!
연구용 시약 및 표준품(표준물질) 전문제조사 Veeprho 공식 수입/공급 업체 코아사이언스 입니다.
자세한 상품 정보 및 관련 문의는 전화 02-858-0328 또는 info@coresciences.co.kr로 문의주시기 바랍니다.
감사합니다!
Azithromycin EP Impurity C
Cat.# VL186009 / Size: inquire
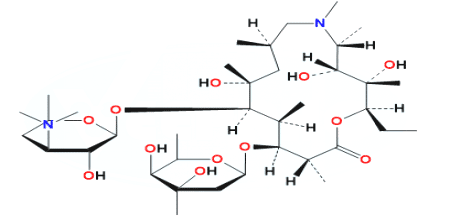
Also known as 3”-O-demethylazithromycin, it is an impurity of Azithromycin that is an azalide, a subclass of macrolide antibiotics drugs that may be bactericidal or bacteriostatic. It is utilized in the treatment of bacterial upper and lower respiratory tract infections, skin structure infections, and sexually transmitted diseases.
Chang, Yan, et al. Factors Influencing the HPLC Determination for Related Substances of Azithromycin.” Journal of Chromatographic Science, Aug. 2015, p. bmv127, https://doi.org/10.1093/chromsci/bmv127. Miguel, L., and C. Barbas. LC Determination of Impurities in Azithromycin Tablets.” Journal of Pharmaceutical and Biomedical Analysis, vol. 33, no. 2, Sept. 2003, pp. 211–17, https://doi.org/10.1016/s0731-7085(03)00258-9.
*본 상품은 오직 연구용으로만 사용 가능합니다. 인체 및 제품화에 사용하실 수 없습니다.

코아사이언스 coresciences Veeprho Korea 한국 대리점 의약품 표준물질 impurity 불순물 유연물질 성분 분석 표준품 지표물질 표준성분 지표성분 유효성분 HPLC 분석 유연물질 reference standards working standards impurity standards chemicals APIs isotope labelled compounds metabolite Veeprho Pharmaceuticals s.r.o., Europe Veeprho Research inc., USA Veeprho Life Sciences UK Ltd, UK Impurity isolation from API or drug products by Preparative HPLC Synthesis of Impurities/Metabolite Intermediates and API
'APIs & Impurities' 카테고리의 다른 글
- Total
- Today
- Yesterday
- OPV
- gradient gel
- 면역화학분석
- 오토클레이브백
- 파라핀 블럭
- time release pellets
- 조직절편제작
- matrix-driven delivery pellet
- 형광염색
- 코아사이언스
- 파라핀 블록
- 막필터
- 후나코시
- 바이오헤저드백
- filter
- Sterlitech
- 연속절편
- 콘드로이틴 황산 올리고당
- allview PAGE buffer
- Coresciences
- 조직절편
- 조직염색절편제작
- solar cells
- 건스터바이오텍
- 고수용성 콘드로이틴
- 미니멸균백
- Funakoshi
- OLED
- material science
- 바이오하자드백
| 일 | 월 | 화 | 수 | 목 | 금 | 토 |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 25 | 26 | 27 | 28 | 29 | 30 | 31 |
